The journey to net zero
In November 2020, UK water companies unveiled a plan to deliver a net-zero water supply for customers by 2030. The sector has achieved significant carbon reductions over the past ten years, as can be seen in the graph below.
UK water sector historic greenhouse gas emissions
Source: Net Zero 2030 Routemap, Water UK
The graph shows that Scope 2 greenhouse gas emissions make the largest contribution. At the same time, this area is where the biggest reductions are being made because water companies are switching to renewable energy sources.
Scope 1 and 3 emission levels have not yet reduced significantly and now represent a substantial proportion of the water industry’s carbon footprint.
One of the biggest contributors to Scope 3 emissions is the chemicals used in water treatment. A lot of chemical production is energy intensive and involves using non-renewable raw materials. The water sector’s drive to net zero therefore goes hand-in-hand with the imperative to reduce the use of chemicals.
Definitions of Scope 1, 2 and 3 emissions
Scope 1: Emissions from sources that an organisation owns or controls directly – for example, from burning fuel in its processes or vehicles.
Scope 2: Emissions that an organisation causes indirectly from the energy it purchases and uses – for example, the energy generated from the need to power a treatment works.
Scope 3: Emissions that are not produced by the organisation itself, or the result of activities from assets owned or controlled by the organisation, but by those it is indirectly responsible for throughout its value chain – for example, from the production of materials used in construction or operations.
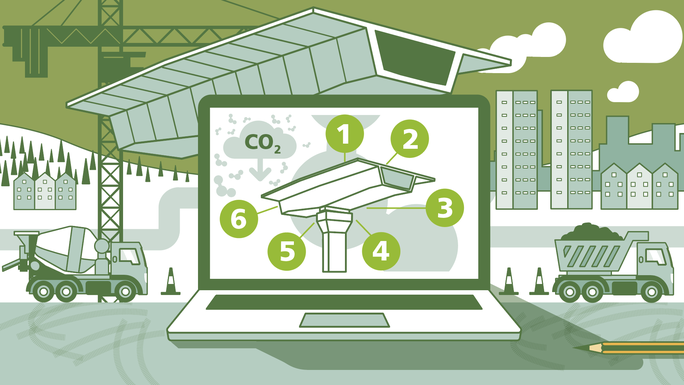
The drinking water treatment process
The process of converting raw water into drinking water involves several stages that typically include those shown in the graphic below.
Adapted from: Combest T, Municipal water treatment process
The use of chemicals in water treatment
Chemicals associated with carbon are added at various stages throughout the treatment process. These stages are explained below:

Coagulation and floculation
In this process, a chemical is added that reacts with the natural alkalinity of the water to form an insoluble precipitate. Various chemicals can be used as coagulants, the most common of which are compounds of aluminium or iron, such as aluminium sulphate (or alum), ferrous sulphate, ferric chloride and sodium aluminate. The main factors that influence the choice of coagulant are availability and affordability.

Disinfection
Once the water has been through the filtration process, it is about as clear and clean as it can be. However, this water may still contain bacteria and viruses. These are destroyed by disinfection, usually through chlorination. Chlorine comes in many different forms, including chlorine gas, sodium hypochlorite (a liquid), or through onsite electro-chlorination. Water companies monitor chlorine levels continuously to ensure there is enough chlorine to disinfect the water, but not enough to cause taste or odour problems.

pH balancing
UK water quality regulations specify that the pH of tap water should be between 6.5 and 9.5. Similar standards exist elsewhere: guidelines for Canadian Drinking Water Quality suggest that the pH should be between 7.0 and 10.5, while in the US the Secondary Maximum Contaminant Level for pH is between 6.5 and 8.5. The Indian Standard for Drinking Water also gives an acceptable standard of 6.5 to 8.5.
If the pH is too low, the water can have a bitter, metallic taste and corrosion that has built up on metal pipes can be released, which could cause health problems. If it is too high, the water will have a slippery feel, a soda-like taste, and leave scale deposits on plumbing and fixtures.
In the UK, the pH of source water varies considerably. In lowland areas, such as the south of England, it tends to be hard (high pH), so water companies add sulphuric acid (H₂SO₄) to reduce the pH. In northern areas with peaty soils, such as Yorkshire, Northumbria and Scotland, water is naturally acidic and an alkaline chemical such as calcium hydroxide (slaked lime) is added to increase the pH.
Adding a coagulant at the start of the treatment process reduces the pH value of the incoming water, so chemicals may be needed to counteract this. As a result, very little pH correction may be needed in some parts of the south of England.

Lead solubility
In the past, lead was used widely for plumbing, and many older households still have lead pipes. In hard water (high pH areas), limescale build-up can prevent lead from dissolving into the water, so lead levels are usually low. However, most water companies add a small amount of orthophosphoric acid to reduce lead levels even further.
Lower-chemical/lower-carbon alternatives
The water industry is working to find lower-carbon alternatives to some of the most carbon-intensive chemicals used to treat water for drinking. These solutions include alternative chemicals that have a lower-carbon footprint in their production and usage, as well as nature-based solutions that have little or no chemical input. Options are being developed and trialled across the treatment process.
Coagulation
“The coagulation process at the beginning of treatment enables some of the organic material to be taken out, but also microbiological material like cryptosporidium [a microscopic parasite that causes the diarrheal disease cryptosporidiosis],” explains Jonathan Bishop, principal process engineer at consultancy Mott MacDonald.
Historically, the chemicals used as coagulants were produced as by-products of heavy industry, such as steel production. However, in countries where this type of industry has declined, the compounds now have to be manufactured specifically for water treatment – a process that generates carbon emissions.
There are low/zero-carbon alternatives available, including slow sand filters (SSFs), in which the source water flows through a layer of sand, where it not only gets physically filtered but is biologically treated to remove both sediments and pathogens. A microbial community quickly establishes itself on the top layer of the sand, making it biologically active, with most of the community being predatory bacteria that feed on water-borne microbes and remove them from the water naturally. The process has been used for more than 150 years and is still used by Thames Water for some of London’s water.
The biggest problem with SSFs is that the filtration beds require a lot of land, which is difficult to find and expensive in highly populated industrialised countries. Where this is not an issue, it is a cost-effective and low-carbon method of removing particles and pathogens from water.
Another option for reducing the use of chemicals at the coagulation and flocculation stage is to use ceramic membranes to remove suspended solids, particles and bacteria – including protozoa such as cryptosporidium. At present, membranes are usually used in combination with coagulation, but research is under way to explore whether they can offer a chemical-free alternative.
Water companies are also looking at ion exchange as an alternative to coagulation for removing organics such as calcium, magnesium, chlorides and nitrates. The process uses polymer beads arranged in a resin bed to exchange these ‘problematic’ dissolved ions in water for less problematic ions.

Slow sand filters (SSFs) are a low/zero-carbon alternative in which the source water flows through a layer of sand, where it not only gets physically filtered but is biologically treated to remove both sediments and pathogens
pH balancing
One of the substances most commonly used to increase pH is calcium hydroxide, or slaked lime, the manufacture of which involves heating limestone to very high temperatures. “It is cheap to mine limestone and slake it, so if you are not thinking about the environmental cost, it is the cheapest thing to use,” Bishop says. “But it has a very high natural carbon footprint from mining and releasing CO2.”
A lower-carbon option is sodium hydroxide (caustic soda), which, while being more expensive to produce, has other benefits. “Lime is only sparingly soluble,” explains Bishop. “You take a full silo of lime and have to make it into a slurry, which is hydroscopic, so things can get clogged up. It is a much cheaper product than sodium hydroxide, but it requires a lot of operation and maintenance. In contrast, sodium hydroxide is delivered as a liquid, so the process is much simpler.”

While being more expensive to produce, sodium hydroxide (caustic soda) is a lower-carbon option – it is delivered as a liquid so the process is much simpler than with slaked lime
Disinfection
Chlorine is the most commonly used disinfectant. It is usually delivered as a gas or a liquid, in the form of sodium hypochlorite. However, another option is onsite electro-chlorination. This is done by electrolysing a brine solution: at one electrode the process generates hydrogen, and at the other it forms oxygen and sodium hydroxide or sodium hypochlorite. “In electro-chlorination, the carbon footprint is associated with the electricity source you are using, so if there is a real demand for zero carbon, it can be done using renewable energy,” Bishop explains.

Onsite electro-chlorination is another option to chlorine. A brine solution is electrolysed – the carbon footprint is associated with the electricity source used
Catchment management
The role of some of the chemical processes used in the production of clean drinking-quality water is to remove impurities that are in the water when it arrives at the treatment works. These can include nitrates and other chemicals that come from fertilisers as well as pesticides that run off into rivers upstream of abstraction points.
Rather than expecting water companies to remove these substances, which adds to the chemical footprint of the treatment plant, an alternative is to tackle the problem at the source and stop them entering the water. “It’s a societal issue,” says Bishop. “The best thing is to prevent the risk in the first place and intervene at a catchment level rather than putting more treatment in."
The move to cut carbon-intensive chemicals from the water treatment process will change the way treatment works are laid out and the facilities that are required. Engineers involved in the design, construction, maintenance and refurbishment of treatment works will in future be asked to accommodate different technologies and incorporate space for nature-based solutions.

Intervening at a catchment level and tackling the problem at source is the best approach
Case study: Church Wilne, Derbyshire
UK water company Severn Trent has embarked on a project that will increase water supplies by up to 93m litres per day with lower-carbon impact and chemical treatment than existing supply sources.
The project centres on a new water treatment works in the small village of Church Wilne in Derbyshire, and a variety of natural and technological solutions, including floating wetlands in three existing gravel beds at the abstraction site on the River Trent.
Case study from Green Recovery Report 2021-22, Severn Trent
WTW = Water treatment works
These floating reed beds will provide the first stage of water treatment and increase biodiversity. When used in combination with ceramic membranes, they will be “a real game-changer in reducing chemical treatment and energy consumption and developing more sustainable treatment solutions”, according to the water company.
The floating wetlands will rise and fall with the level of the river and can be removed and replaced relatively easily from the gravel beds, making ongoing maintenance much easier.
Other new treatment technologies will be trialled at a pilot plant being built at Church Wilne, which is a scaled down version of the new water treatment works that will be operated for 12 months to test the technologies that are proposed for the full-scale works. These new technologies include SIX – Suspended Ion Exchange – a process developed by Pure Water & Nature Technologies for removing unwanted organic material, as well as ceramic membranes and granular activated carbon for filtration.
Sign up to receive news from ICE Knowledge direct to your inbox.